Date: 23/12/2022
Relevance: GS-3: Conservation, environmental pollution and degradation, environmental impact assessment.
Key Phrases: Great lakes, Acidity of Continental lakes, Lake Huron project, Thunder Bay National Marine Sanctuary, Ocean acidification, Impact of ocean acidification.
Context:
- A team of scientists is working on building a sensor network to detect the trends in the water chemistry of Lake Huron, one of the five Great Lakes of North America.
- It is the first step towards developing a system that would be capable of measuring the carbon dioxide and pH levels of the Great Lakes over several years.
- Researchers hope the data from the Lake Huron project would add to scientific information on the subject.
Key Highlights
- It is well known that the increase in atmospheric carbon dioxide has caused the world’s oceans to turn more acidic but lakes were not given attention.
- Recently, it has been observed that by 2100 the Great Lakes might approach acidity at around the same rate as the oceans.
- The Lake Huron Study-Project
- Two sensors have been attached to a floating weather buoy at Thunder Bay National Marine Sanctuary near Alpena, Michigan, in the US.
- One of them measures carbon dioxide pressure in the water column and the other one the pH.
- Crews are also collecting water samples at varying depths within an 11,137-sq km area for chemical analysis.
The Great lakes
- The Great Lakes are five interconnected bodies of water straddling the US-Canada border that drain into the Gulf of St Lawrence in the North Atlantic through the St Lawrence River.
- They are the largest group of freshwater lakes in the world.
- The Great lakes (in area wise descending order) comprise Superior, Huron, Michigan, Erie, and Ontario.
- The US-Canada border passes through Lakes Superior, Huron, Erie, and Ontario.
- Lake Michigan lies entirely in the US.
- Lake Superior is the largest continental lake in the world by area.
- Lakes Michigan and Huron are sometimes considered as a single water
body.
- When taken together, they are the world’s largest freshwater lake by surface area.
- By itself, Lake Huron is the world’s third largest freshwater lake, after Lake Superior and Lake Victoria.
- Significance
- According to NOAA the Great Lakes contain a fifth of the world’s total freshwater, and are a crucial source of irrigation and transportation.
- The Great Lakes also serve as the habitat for more than 3,500 species of plants and animals.

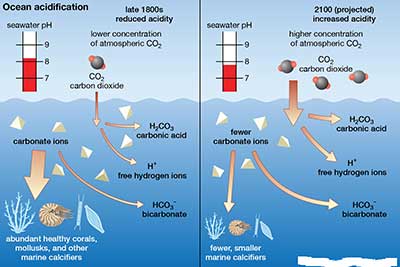
Acidification of water bodies
- Acidification of oceans or freshwater bodies takes place when excess carbon dioxide in the atmosphere gets rapidly absorbed into them.
- An estimated 30–40% of the carbon dioxide from human activity released into the atmosphere dissolves into oceans, rivers, and lakes.
- According to the National Oceanic and Atmospheric Administration (NOAA) of the US government, oceans have 200 years alone, ocean water has become 30 percent more acidic.
- Process of acidification
- The excessively dissolved Carbon dioxide (CO2) reacts with water molecules (H2O) to establish chemical equilibrium.
- This reaction of CO2 with water forms the weak acid H2CO3 (carbonic acid).
- Most of this acid dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3-) which leads to increase in H+ ions.
- As the H+ ions increase it reduces pH (a measure of acidity) of the water and the oceans acidify, that is they become more acidic or rather less alkaline. This process is called ocean acidification.
Causes of acidification
- Disposal of waste such as domestic, industrial, sewage disposal and agricultural waste contributes to ocean acidification and other water bodies.
- Increased Fossil Fuel usage such as coal, petroleum, etc. release a large amount of carbon dioxide in the atmosphere which leads to acidification.
- High rates of deforestation have shrunk the Carbon Sinks and they are not absorbing the high amount of CO2.In fact parts of Amazon forests have become source of CO2. This leads to increased acidification.
Consequences of Acidification
- Alarming low levels of pH
- It is estimated that by the end of this century the surface waters of the ocean could have a pH of around 7.8.
- The last time the ocean pH was this low was during the middle Miocene, 14-17 million years ago.
- Impact on biodiversity and food security
- Ocean acidification is currently affecting the entire ocean, including coastal estuaries and waterways.
- Billions of people worldwide rely on food from the ocean as their primary source of protein thus it will jeopardize food security.
- Economic losses and unemployment
- Many jobs and economies around the world depend on the fish and shellfish that live in the ocean so there will be an impact on the economy and the jobs associated with it.
- Coral reefs are under threat
- Carbonate is the main requirement of corals to make their internal structure, but ocean acidification is causing the reduction of carbonate ions in the water.
Estimated Impact of the Acidification on the lakes
- According to NOAA’s Ocean, Coastal, and Great Lakes Acidification
Research Plan 2020
- Based on assumptions of current projections of anthropogenic carbon dioxide and constant alkalinity the five lakes would witness a pH decline of 0.29-0.49 pH units.
- It means they would become more acidic — by 2100.
- Acidification may lead to a decrease in native biodiversity, create physiological challenges for organisms, and permanently alter the structure of the ecosystem.
- It would also severely impact the hundreds of wooden shipwrecks that are believed to be resting at the bottom of these lakes.
- According to a 2018 study of four German reservoirs at Ruhr
University Bochum found that their pH levels had declined three times
faster in 35 years than in oceans since the Industrial Revolution.
- As a result of the increase in acidity, the ability of water fleas to defend themselves against predators was compromised.
- It is expected that a similar trend might be seen in the Great Lakes as well.
Way forward
- Scientists believe that without a collective global effort to reduce concentrated atmospheric carbon dioxide, not much can be done to stop the acidification of the Great Lakes.
- So if we can cut our global warming emissions, and limit future warming, we can significantly reduce the harm to marine ecosystems.
- Therefore the most effective way to limit acidification of water bodies including the great lakes is to act on climate change, implementing solutions to dramatically reduce the use of fossil fuels.
Source: Indian Express
Mains Question:
Q. What do you understand by ocean acidification? Analyze the phenomenon of acidification of continental aquatic systems such as freshwater lakes. (250 words)






















