Context:
On June 26, Nature published a paper by researchers at the University of California, Berkeley, and the Arc Institute in the U.S. describing a new RNA-guided gene editing system.
The Discovery of Transposons
Understanding of Genetics
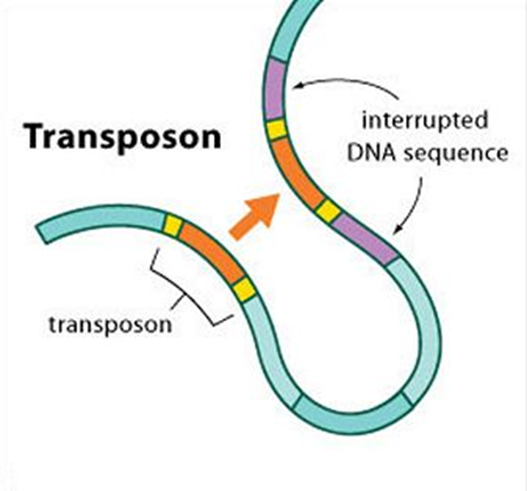
Transposable elements (TEs), also known as "jumping genes," are DNA sequences that move from one location on the genome to another. The discovery of transposons revolutionised our understanding of genetics, particularly their role in enabling nature’s wondrous diversity. In 1948, Barbara McClintock at the Carnegie Institution challenged the prevailing concept that genes are stable and orderly arranged on chromosomes. She discovered that some genes, called mobile elements or transposons, could move around within the genome.
Jumping Genes
McClintock also observed that these mobile elements could reversibly alter gene expression depending on their insertion locations, using corn kernel colours as a surrogate to understand hereditary characteristics. For this groundbreaking work, she was awarded the Nobel Prize in Physiology or Medicine in 1983. Between 1948 and 1983, researchers found transposons in various life forms, including bacteriophages, bacteria, plants, worms, fruit flies, mosquitoes, mice, and humans, earning them the nickname ‘jumping genes.’
Types of Transposons
Today, scientists know that there are many different types of TEs, as well as a number of ways to categorize them. One of the more common divisions is between those TEs that require reverse transcription (i.e., the transcription of RNA into DNA) in order to transpose and those that do not. The former elements are known as retrotransposons or class 1 TEs, whereas the latter are known as DNA transposons or class 2 TEs. The Ac/Ds system that McClintock discovered falls in the latter category. Different classes of transposable elements are found in the genomes of different eukaryotic organisms.
‘Sleeping Beauty’ Transposon
What Jumping Genes Do?
Transposons significantly influence gene effects by turning their expression “on” or “off” through various epigenetic mechanisms, making them tools of evolution due to their ability to rearrange the genome and introduce changes. Over 45% of the human genome consists of transposable elements. While they create diversity, they can also cause gene mutations leading to diseases. However, most transposons have inherited mutations and become inactive, unable to move within the genome.
Reviving Inactive Transposons for Biomedical Applications
Researchers have been attempting to resurrect inactive transposons from animal genomes for potential biomedical applications, such as genetic correction to cure diseases or for gene therapy. For instance, in 1997, researchers reconstructed a transposon called “sleeping beauty” at the molecular level from fish genomes. This transposon had been dormant in vertebrates for millions of years. Researchers reprogrammed this synthetic transposon to work in human cells, hoping that similar transposons might, in the future, turn off problematic genes or over-express beneficial ones.
RNA-Guided Transposons
Transposons Can Encode siRNAs That Mediate Their Own Silencing
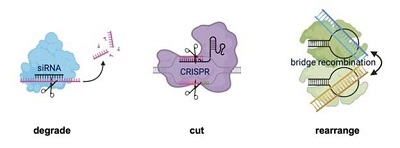
On June 26, researchers from the University of California, Berkeley, and the Arc Institute in the U.S. published a paper in Nature describing a new RNA-guided gene editing system. This tool builds on an earlier discovery that one of the genes in the IS110 family of bacterial transposons contains instructions for cells to make an RNA molecule with two loops. This RNA can bind to two DNA pieces instead of the usual one, forming a bridge between them.
Bridge RNA with High Efficiency and Specificity
The researchers used this bridge RNA to edit DNA. The RNA’s two loops can independently bind to two separate DNA pieces: one loop identifies the target site in the genome to be altered, and the other specifies the DNA to be inserted. Each loop is independently programmable, allowing researchers to mix and match any target and donor DNA sequences. In their study, the researchers reported that in Escherichia coli bacteria, the bridge RNA had more than 60% insertion efficiency (ability to introduce a desired gene) and a 94% specificity (ability to target the intended location on the genome).
Boon for Synthetic Biology
Researchers from the University of Tokyo have detailed the mechanisms of genome modification using bridge RNA. Studying IS110 transposons with cryo-electron microscopy, they found that these transposons work as dimers, where one copy binds to the target DNA and the other to the donor DNA, bridged by RNA. This method offers advantages over CRISPR, as it ensures clean, specific cuts without leaving nucleotide errors.
This precision allows for the addition, deletion, or inversion of DNA sequences of any length, making it possible to insert functional copies of faulty genes into any genome location. This technique is especially beneficial for synthetic biology and for treating genetic diseases by replacing faulty genes or correcting chromosomal inversions and deletions.
Conclusion
Transposons influence gene effects by turning their expression ‘on’ or ‘off’ and can rearrange the genome, thus enabling nature’s diversity. Researchers have reconstructed a transposon called ‘sleeping beauty’ from fish genomes, which had been dormant for millions of years. A similar synthetic transposon may, in the future, allow us to turn off problematic genes or over-express beneficial ones. A new RNA-guided gene editing system using bacterial transposons can treat various genetic diseases by replacing a functional gene copy in a specific genomic location and may also address chromosomal inversions or deletions.
|
Probable Questions for UPSC Mains
|
Source: The Hindu