Context:
India's recent public health discourse on HPV vaccination emphasises its role in preventing cervical cancer and related deaths. However, only a few of the 200 HPV strains are linked to precancerous lesions. While most cervical cancer fatalities are HPV-positive, the majority of HPV-positive individuals do not develop cancer.
Human Papilloma Virus (HPV)
- Background : Human papillomavirus (HPV) refers to a group of 200 known viruses, with most infections being asymptomatic. However, high-risk HPV types can lead to genital warts and cancers such as cervical, vulvar, vaginal, penile, and throat cancers. In 2019, HPV caused approximately 620,000 cancer cases in women and 70,000 in men. Prophylactic vaccination and HPV screening effectively prevent these cancers by targeting pre-cancerous lesions.
- HPV is a common sexually transmitted infection that can affect the skin, genital area, and throat. Most HPV infections resolve without treatment, but some cause genital warts or abnormal cell development leading to cancer. The HPV vaccine, which does not contain live virus or DNA, prevents HPV-related cancers but does not treat existing infections. Cervical cancer is the most common HPV-related cancer, with screening tests available to detect and treat precancerous changes early.
- Symptoms : Most people with HPV infections have no symptoms, and the immune system typically clears the infection within one to two years. Some infections cause genital warts, which can be painful, itchy, or bleed. Persistent HPV infection can lead to cervical cell changes and potentially cervical cancer over 15-20 years.
- Development of Indigenous HPV Vaccine : The Serum Institute of India (SII) developed 'Cervavac' and promoted HPV vaccination as an indigenous and affordable vaccine. However, it took nearly two decades for this 'indigenous' vaccine after the introduction of a patented HPV vaccine in the United States and Australia. Cervavac uses virus-like particles (VLPs) produced using recombinant Deoxyribose Nucleic acid (rDNA) techniques to generate an immune response against HPV infections.
Impact of Patenting on Vaccine Development
The U.S. Patent Act amendment in the 1980s allowed the patenting of genetically modified organisms (GMOs) and life processes, drastically changing vaccine development and innovation. The globalisation of U.S. patent laws through the World Trade Organization Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) since 1995 further influenced this change. The first vaccines for cervical cancer, Gardasil by Merck and Cervarix by Glaxo Smithkline, were developed under these new innovation conditions.
- Effects on Indian Pharmaceutical Industry : India's earlier Patent Act (1970) enabled the growth of domestic industries by abolishing product patents and allowing only process patents. This facilitated the production of low-cost generic drugs and vaccines. However, under the current product patent regime, the locally made DNA vaccine against cervical cancer had to wait two decades for the expiry of product patents. Despite the expiry of key patents, the current market price of Cervavac remains exorbitant.
- Pricing and Market Dynamics : Before the domestically manufactured vaccine, Gardasil and Cervarix were sold in India for ₹4,000 a dose. Even at about half that price, Cervavac remains largely unaffordable. The pricing strategy of SII is questionable given the significant funding and shared infrastructure for Cervavac production. The strategy seems to prioritize high margins over low-margin, high-volume trade, essential for public health success.
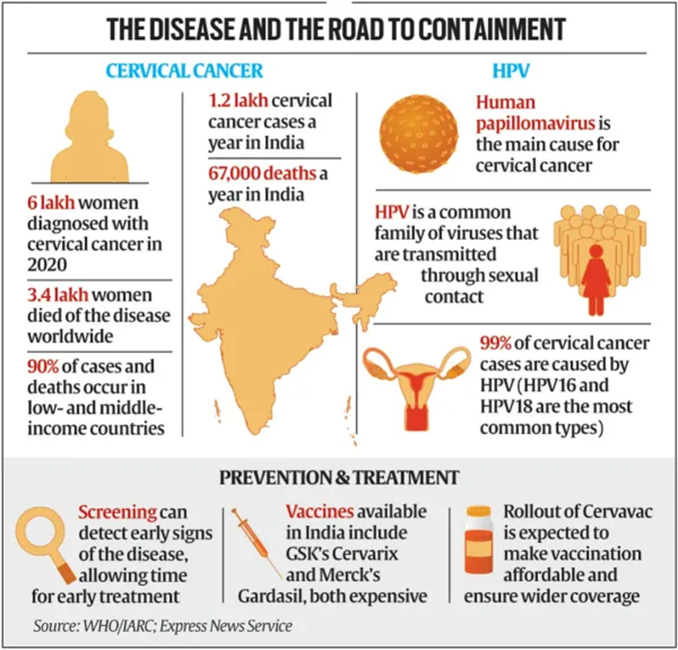
Vaccination Debate
- Declining Trends : The Population Based Cancer Registries (PBCR) of India and the International Agency for Research on Cancer (IARC) acknowledge the declining trends of cervical cancer prevalence globally, regardless of vaccine coverage or efficacy. The push for universal vaccination of girls against HPV overlooks the more justifiable selective vaccination of high-risk groups. The assumption that pre-puberty girls engaging in promiscuous physical relations are a risk factor for the adult population is a moral conundrum in Indian society and reeks of patriarchy.
- Lack of Competing Vaccines : The unavailability of competing vaccines from domestic players is a serious concern. Despite the expiry of earlier patent barriers, several domestic players' HPV vaccine candidates have not entered the market, raising concerns about competition and pricing.
- Government Vaccination Programme and Public Health Implications : Cervavac is recommended under the government vaccination programme for girls aged nine to 26 at ₹500 for two doses, which is expensive even for the government. The retail price of Cervavac will increase four-fold for those not covered by the government programme, posing a significant financial burden in a country with low insurance penetration and high out-of-pocket health expenditures.
Solutions for Affordable and Effective HPV Vaccination in India
- Promote Competition in HPV Vaccine Market: The government can incentivize and support research and development (R&D) efforts of domestic pharmaceutical companies to develop competing HPV vaccines. This will create a competitive market, potentially leading to lower prices. India's legal framework allows compulsory licensing under specific circumstances. This could be explored as a last resort to ensure access to affordable vaccines if competition remains stagnant.
- Revise Pricing Strategy for Cervavac: SII should consider a cost-plus pricing model for Cervavac, ensuring a reasonable profit while keeping the vaccine affordable for the public and government programs. The government could explore providing subsidies to SII or implementing price controls to make Cervavac more accessible.
- Targeted Vaccination and Public Awareness Campaigns: Instead of universal vaccination, prioritize high-risk groups identified through screening programs. This would optimize resource allocation and address concerns about unnecessary vaccination. Conduct public awareness campaigns highlighting the benefits of HPV vaccination while addressing societal concerns and promoting gender equality.
- Strengthen Public Health Infrastructure: Increase awareness and accessibility of HPV vaccination programs across the country, particularly in rural areas. Invest in screening programs: Regular cervical cancer screening programs for women above a certain age can detect and treat pre-cancerous lesions before they develop into cancer.
- Leverage International Collaboration: Explore partnerships with international organizations or vaccine manufacturers for technology transfer to facilitate local production of HPV vaccines. Collaborate with international bodies for research grants and knowledge sharing to accelerate the development of new and improved HPV vaccines.
Conclusion
The need for universal HPV vaccination to prevent cervical cancer remains debated, and the lack of competition and opaque pricing strategies in the HPV vaccine market merit investigation in the public interest. But by implementing these solutions, India can achieve a more balanced and effective approach to HPV vaccination. Prioritising affordability, competition, and targeted vaccination strategies can ensure better public health outcomes and reduce the burden of cervical cancer.
|
Probable Questions for UPSC Mains
|
Source: The Hindu