Context:
India’s National Security Advisor, Ajit Doval, and the US National Security Advisor, Jake Sullivan, have recently reviewed potential areas of cooperation under the Initiative on Critical Emerging Technologies (iCET). Biotechnology was identified as a key area of cooperation, both for its significant economic potential and its critical importance from a security perspective. However, the initiative has yet to define specific bilateral cooperation agendas in biotechnology, highlighting the need to understand the current state of biosecurity and biosafety cooperation, its significance, and future directions.
The Imperative for Robust Biosecurity and Biosafety
- Robust Pathogen Surveillance and Regulation : The COVID-19 pandemic has underscored the need for robust pathogen surveillance systems, strict regulation of critical biological research areas, and a global, multidisciplinary approach to managing diseases and biological disasters. Biosecurity focuses on protecting against biological threats, while biosafety involves regulations to prevent laboratory accidents and accidental pathogen release.
- Opportunities for Collaboration Under iCET : The United States has launched a Biosafety and Biosecurity Innovation Initiative under an executive order, aimed at advancing life sciences research while addressing associated vulnerabilities and risks. In contrast, India introduced the Public Health (Prevention, Control and Management of Epidemics, Bio-terrorism and Disasters) Bill in 2017, though its current status and contents remain unclear.
Given the aftermath of the pandemic, developing formal institutional mechanisms for biosecurity and biosafety is crucial. The iCET presents an opportunity for the US and India to collaborate on these pressing issues, fostering an open, accessible, and secure technology ecosystem based on mutual trust and democratic values. This collaboration will involve bridging technological efforts among governments, businesses, and academic institutions, and facilitating technological innovation while easing regulatory barriers.
Current Status of Biotechnology Cooperation
- One Health Approach : Biotechnology cooperation between the US and India is currently managed through the 1.5-Track Biosecurity Dialogue, jointly hosted by the John Hopkins Centre for Health Security and the Regional Centre for Biotechnology, an autonomous institution of India’s Department of Biotechnology. This dialogue has identified key areas of cooperation, including increased bilateral engagement, a “One Health” approach to emerging biological threats, measures against misinformation campaigns, and laboratory safety. This year’s dialogue is scheduled to convene in New Delhi, although exact dates are yet to be finalized.
- Workshops and Legislative Developments under iCET : The US National Academies of Sciences, Engineering and Medicine will host a workshop series on biotechnology cooperation under iCET, focusing on training in infectious disease management and biosafety methodologies. Additionally, the US is set to pass a new Biosecure Act, aimed at reducing reliance on China for pharmaceutical needs due to national security concerns. If enacted, this legislation will offer significant opportunities for Indian Contract Development and Manufacturing Organizations (CDMOs), with iCET serving as a platform for developing risk assessment frameworks in the pharmaceutical sector. Despite these efforts, there remains ample opportunity to enhance biosecurity and biosafety through iCET.
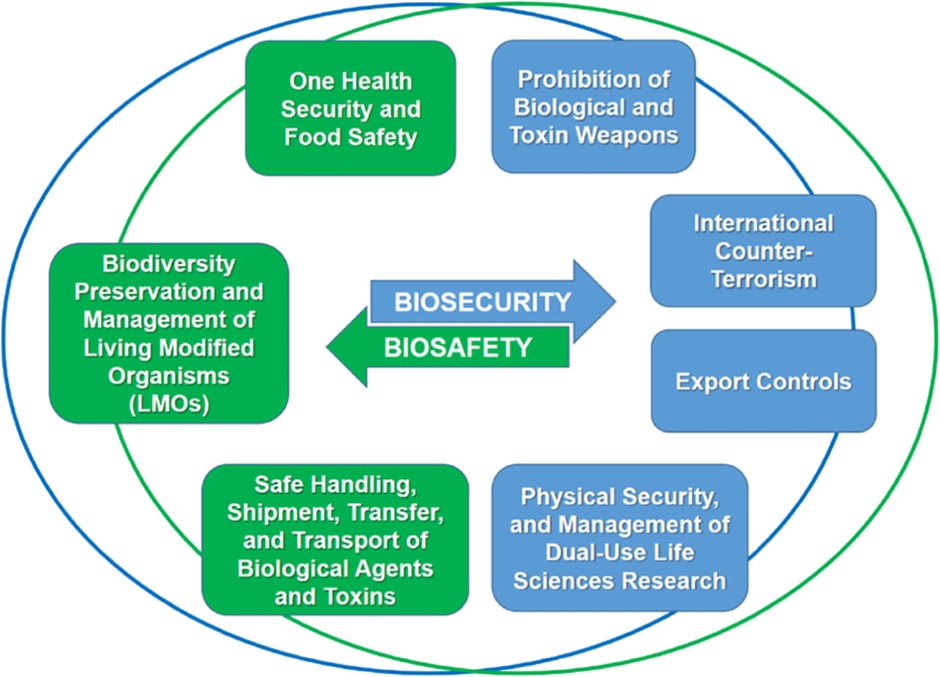
Dual-Use Research Concerns
- Advancements and Risks in Biotechnology : Biotechnology advancements have led to significant progress in vaccines, diagnostics, drugs, and medical devices. However, there are concerns about the ‘dual-use dilemma,’ where life science research, while beneficial, may also be misused to cause harm. For example, synthetic biology has enabled the development of antibodies and mRNA vaccines, but it also poses risks if exploited by malicious actors to create harmful biological agents. This highlights the need for mechanisms to identify and manage potential risks associated with life science technologies while maximizing their societal benefits.
- Collaborative Approaches for Risk Management and Safety : Developing risk and innovation frameworks requires collaboration among researchers, academia, the private sector, security experts, public health professionals, and think tanks. As biotechnology advances, researchers and academics must recognize the dual-use potential of their work, while security experts need to understand how these technologies might be misused. This is particularly relevant for the pharmaceutical industry, which faces dual-use concerns with new vaccine platforms. Collaborative approaches between biotechnology and security communities are essential, with opportunities for joint workshops and training under iCET to enhance laboratory safety and proper handling of biological materials.
Disease Surveillance and Early Warning Systems
- Strengthening Health Surveillance and the Need for US-India Collaboration : The COVID-19 pandemic exposed limitations in health surveillance systems, emphasizing the need for US-India collaboration to strengthen health intelligence networks for potential biological outbreaks, disasters, or terrorist attacks. The ongoing H5N1 avian influenza epidemic in the US and Nipah virus outbreaks in India, exacerbated by factors like climate change and population growth, highlight the necessity of a “One Health” approach to biosecurity. Understanding zoonotic viruses’ ability to cross species barriers and anthropogenic factors driving spillover events is crucial.
Enhancing Health Intelligence Networks and Expanding Institutional Roles
Collaborative efforts between the US Centers for Disease Control and Prevention (CDC) and India’s National One Health Mission can enhance responses to infectious diseases in humans, animals, and plants. Joint initiatives could establish Indo-US centers for environmental surveillance, including wastewater and forest ecosystems, and generate real-time information on outbreaks. Improving health intelligence networks through systematic data collection and analysis can aid early detection and response to health emergencies.
Expanding the roles of institutional bodies like the National Centre for Medical Intelligence (US) and the Central Bureau of Health Intelligence (India) to address unknown biological threats through microbial forensics and threat characterization is vital. Additionally, biosecurity efforts should extend to cybersecurity collaborations, protecting health systems information from malicious actors.
Conclusion
Rapid advancements in biotechnology necessitate a robust regulatory framework to ensure biosecurity and biosafety. Partnerships between American and Indian private sectors and regulatory bodies can develop schemes to assess biosecurity risks associated with novel biotechnologies. Knowledge transfer mechanisms can educate both biotechnology and security communities. Collaborative health intelligence networks can provide valuable insights into transnational biological threats. iCET represents an ideal platform for addressing dual-use research, disease surveillance, and other biosecurity issues through industrial and academic partnerships, pandemic-preparedness exercises, and training programs, ultimately strengthening the biosecurity framework.
|
Probable Questions for UPSC Mains
|
Source: ORF India







